Geological time, relative dating and radiometric dating
age of earth
t the age of the Earth is approximately 4.54 ± 0.05 billion years (4.54 × 109 years ± 1%).This dating is based on evidence from radiometric- age dating of meteorite material and is consistent with the radiometric ages of the oldest-known terrestrial and lunar samples
Geological processes
Geological processes are dynamic processes at work in the earth's landforms and surfaces. The mechanisms involved, weathering, erosion, and plate tectonics, combine processes that are in some respects destructive and in others constructive.
relative dates based on cross-cutting relationships
Cross-cutting relationships is a principle of geology that states that the geologic feature which cuts another is the younger of the two features. It is a relative dating technique in geology.
There are several basic types of cross cutting relationships:
- Structural relationships may be faults or fractures cutting through an older rock.
- Intrusional relationships occur when an igneous pluton or dike is intruded into pre-existing rocks.
- Stratigraphic relationships may be an erosional surface (or unconformity) cuts across older rock layers, geological structures, or other geological features.
- Sedimentological relationships occur where currents have eroded or scoured older sediment in a local area to produce, for example, a channel filled with sand.
- Paleontological relationships occur where animal activity or plant growth produces truncation. This happens, for example, where animal burrows penetrate into pre-existing sedimentary deposits.
- Geomorphological relationships may occur where a surficial feature, such as a river, flows through a gap in a ridge of rock. In a similar example, an impact crater excavates into a subsurface layer of rock.
Radiometric dating or radioactive dating
is a technique used to date materials such as rocks or carbon, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares the abundance of a naturally occurring radioactive isotope within the material to the abundance of its decay products, which form at a known constant rate of decay. The use of radiometric dating was first published in 1907 by Bertram Boltwood and is now the principal source of information about the absolute age of rocks and other geological features, including the age of fossilized life forms or the age of the Earth itself, and can also be used to date a wide range of natural and man-made materials.
Together with stratigraphic principles, radiometric dating methods are used in geochronology to establish the geologic time scale. Among the best-known techniques are radiocarbon dating, potassium–argon dating and uranium–lead dating. By allowing the establishment of geological timescales, it provides a significant source of information about the ages of fossils and the deduced rates of evolutionary change. Radiometric dating is also used to date archaeological materials, including ancient artifacts.
Fundamentals of radiometric dating
Radioactive decay
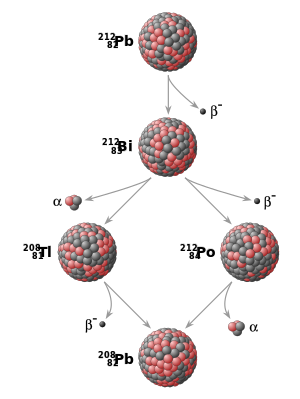
All ordinary matter is made up of combinations of chemical elements, each with its own atomic number, indicating the number of protons in the atomic nucleus. Additionally, elements may exist in different isotopes, with each isotope of an element differing in the number of neutrons in the nucleus. A particular isotope of a particular element is called a nuclide. Some nuclides are inherently unstable. That is, at some point in time, an atom of such a nuclide will undergo radioactive decay and spontaneously transform into a different nuclide. This transformation may be accomplished in a number of different ways, including alpha decay (emission of alpha particles) and beta decay (electron emission, positron emission, or electron capture). Another possibility is spontaneous fission into two or more nuclides.
While the moment in time at which a particular nucleus decays is unpredictable, a collection of atoms of a radioactive nuclide decays exponentially at a rate described by a parameter known as the half-life, usually given in units of years when discussing dating techniques. After one half-life has elapsed, one half of the atoms of the nuclide in question will have decayed into a "daughter" nuclide or decay product. In many cases, the daughter nuclide itself is radioactive, resulting in a decay chain, eventually ending with the formation of a stable (nonradioactive) daughter nuclide; each step in such a chain is characterized by a distinct half-life. In these cases, usually the half-life of interest in radiometric dating is the longest one in the chain, which is the rate-limiting factor in the ultimate transformation of the radioactive nuclide into its stable daughter. Isotopic systems that have been exploited for radiometric dating have half-lives ranging from only about 10 years (e.g., tritium) to over 100 billion years (e.g., samarium-147).[4]
For most radioactive nuclides, the half-life depends solely on nuclear properties and is essentially a constant. It is not affected by external factors such as temperature, pressure, chemical environment, or presence of a magnetic or electric field.[5][6][7] The only exceptions are nuclides that decay by the process of electron capture, such as beryllium-7, strontium-85, and zirconium-89, whose decay rate may be affected by local electron density. For all other nuclides, the proportion of the original nuclide to its decay products changes in a predictable way as the original nuclide decays over time. This predictability allows the relative abundances of related nuclides to be used as a clock to measure the time from the incorporation of the original nuclides into a material to the present.
Accuracy of radiometric dating
The basic equation of radiometric dating requires that neither the parent nuclide nor the daughter product can enter or leave the material after its formation. The possible confounding effects of contamination of parent and daughter isotopes have to be considered, as do the effects of any loss or gain of such isotopes since the sample was created. It is therefore essential to have as much information as possible about the material being dated and to check for possible signs of alteration. Precision is enhanced if measurements are taken on multiple samples from different locations of the rock body. Alternatively, if several different minerals can be dated from the same sample and are assumed to be formed by the same event and were in equilibrium with the reservoir when they formed, they should form an isochron. This can reduce the problem of contamination. In uranium–lead dating, the concordia diagram is used which also decreases the problem of nuclide loss. Finally, correlation between different isotopic dating methods may be required to confirm the age of a sample. For example, the age of the Amitsoq gneisses from western Greenland was determined to be 3.6 ± 0.05 million years ago (MA) using uranium–lead dating and 3.56 ± 0.10 Ma using lead–lead dating, results that are consistent with each other.
Accurate radiometric dating generally requires that the parent has a long enough half-life that it will be present in significant amounts at the time of measurement (except as described below under "Dating with short-lived extinct radionuclides"), the half-life of the parent is accurately known, and enough of the daughter product is produced to be accurately measured and distinguished from the initial amount of the daughter present in the material. The procedures used to isolate and analyze the parent and daughter nuclides must be precise and accurate. This normally involves isotope-ratio mass spectrometry.
The precision of a dating method depends in part on the half-life of the radioactive isotope involved. For instance, carbon-14 has a half-life of 5,730 years. After an organism has been dead for 60,000 years, so little carbon-14 is left that accurate dating can not be established. On the other hand, the concentration of carbon-14 falls off so steeply that the age of relatively young remains can be determined precisely to within a few decades.
Closure temperature
If a material that selectively rejects the daughter nuclide is heated, any daughter nuclides that have been accumulated over time will be lost through diffusion, setting the isotopic "clock" to zero. The temperature at which this happens is known as the closure temperature or blocking temperature and is specific to a particular material and isotopic system. These temperatures are experimentally determined in the lab by artificially resetting sample minerals using a high-temperature furnace. As the mineral cools, the crystal structure begins to form and diffusion of isotopes is less easy. At a certain temperature, the crystal structure has formed sufficiently to prevent diffusion of isotopes. This temperature is what is known as closure temperature and represents the temperature below which the mineral is a closed system to isotopes. Thus an igneous or metamorphic rock or melt, which is slowly cooling, does not begin to exhibit measurable radioactive decay until it cools below the closure temperature. The age that can be calculated by radiometric dating is thus the time at which the rock or mineral cooled to closure temperature. Dating of different minerals and/or isotope systems (with differing closure temperatures) within the same rock can therefore enable the tracking of the thermal history of the rock in question with time, and thus the history of metamorphic events may become known in detail. This field is known as thermochronology or thermochronometry.
- CROSS CUTTING RELATIONSHIP TYPES
- Structural relationships may be faults or fractures cutting through an older rock.
- Intrusional relationships occur when an igneous pluton or dike is intruded into pre-existing rocks.
- Stratigraphic relationships may be an erosional surface (or unconformity) cuts across older rock layers, geological structures, or other geological features.
- Sedimentological relationships occur where currents have eroded or scoured older sediment in a local area to produce, for example, a channel filled with sand.
- Paleontological relationships occur where animal activity or plant growth produces truncation. This happens, for example, where animal burrows penetrate into pre-existing sedimentary deposits.
- Geomorphological relationships may occur where a surficial feature, such as a river, flows through a gap in a ridge of rock. In a similar example, an impact craterexcavates into a subsurface layer of rock




Comments
Post a Comment